Understanding eelgrass population dynamics through paleoecology
Dr. Sandy Wyllie-Echeverria, Friday Harbor Laboratories, University of Washington
Dr. Kendall Valentine, University of Washington
Dr. Bruce Finney, Idaho State University
Seagrasses are a critical part of coastal ecosystems, providing a unique habitat, but have been disappearing at a rapid rate globally. Seagrass distributions are controlled by a number of factors, including sediment characteristics. In this project, we aim to understand how changes in environmental factors control the persistence of the seagrass, Zostera marina, eelgrass, using a paleoecological approach. Our study site, False Bay, San Juan Island, is a shallow water bay that lies within the Salish Sea region of Washington State and is a marine reserve managed by the Friday Harbor Laboratories, University of Washington. We will reconstruct the environmental history of the bay from analysis of sediment cores spanning to the last ice age. Reconstruction of eelgrass abundance will be determined by downcore changes in carbon isotopes (δ13C), a proxy for its abundance. Corresponding sedimentary environments will be determined through grain-size, organic content and other analyses. The sediment and isotope data open a “window into the past” that will allow us to determine favorable conditions for eelgrass, and sequences of changes leading to eelgrass decline.
Coastal Fog and Climate Refugia: Combining photos, temperature sensors, and tide tables with satellite remote sensing
Dr. Jessica Lundquist, Civil and Environmental Engineering, University of Washington
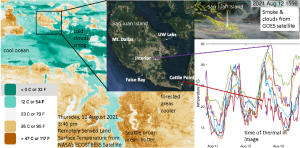
Marine stratus and coastal fog are common along the western U.S. coast, but studies disagree about whether fog frequency is increasing or decreasing. This marine boundary layer is fast-moving, hard to forecast, a hazard for air and sea navigation, and provides huge contrasts in air temperatures during summer heat waves. While most prior studies have focused on California, fog and humidity are more critical to Washington area intertidal organisms due to the timing of the tides. For example, Friday Harbor and Seattle, Washington have over twice as many hours of midday low tide exposure as most California locations (Helmuth et al., 2002). For this project, undergraduate interns will deploy and collect timelapse cameras along with air temperature and relative humidity sensors at coastal and interior sites across San Juan Island, adding data to measurements taken since summer 2021. Interns will analyze this data in the context of regional winds, tides, and heat waves to understand when and where small-scale temperature variations matter the most to local ecology. Interns will work with python programming tutorials to process GOES, VIIRS, and ECOSTRESS satellite data to put the local observations in a regional context and better understand both the accuracy of remote sensing products and what controls the extent and duration of summer temperature peaks and marine cloud cover (see example imagery below, highlighting 15°C (27°F) variation in peak temperatures across San Juan Island on 12 August 2021). Prior programming experience is a plus but not required; anyone with an interest to learn is encouraged to apply.
Reference: Helmut et al. (2002), https://www.science.org/doi/abs/10.1126/science.1076814
Potential impact of marine heatwaves on growth and development of echinoderm larvae
Dr. Sophie George, Biology Department, Georgia Southern University
 Sea stars in the Pacific Northwest have been devastated by sea star wasting disease (SSWD). Because marine heat waves (MHWs) are predicted to increase in duration and intensity, how larvae of these species respond to increasing temperatures has become of interest in our lab. Recent studies investigated whether the swimming behaviors of bipinnariae and brachiolariae of these species differ when exposed to temperatures of 19°C in the presence and absence of food. We discovered that bipinnariae increased their swimming speeds and turning rates at high temperatures. We also discovered that high temperatures accelerate growth and development to metamorphosis. This raises the question as to whether high temperatures lead to trade offs between growth of larval structures and the development of juvenile structures. During summer 2025, students and I will have the opportunity to examine the effects of marine heatwaves (MHWs) on larval morphology, swimming speeds, and juvenile development. Data collected will be analyzed using ImageJ and R software and presented at one or more international conferences. The long-term goal of this project is a better understanding of the potential effects of MHWs on the bipinnaria and brachiolaria larval stages of sea stars in the Pacific Northwest. These results will provide insights into the role these larvae play in the recovery of adult populations devastated by SSWD in recent years.
Sea stars in the Pacific Northwest have been devastated by sea star wasting disease (SSWD). Because marine heat waves (MHWs) are predicted to increase in duration and intensity, how larvae of these species respond to increasing temperatures has become of interest in our lab. Recent studies investigated whether the swimming behaviors of bipinnariae and brachiolariae of these species differ when exposed to temperatures of 19°C in the presence and absence of food. We discovered that bipinnariae increased their swimming speeds and turning rates at high temperatures. We also discovered that high temperatures accelerate growth and development to metamorphosis. This raises the question as to whether high temperatures lead to trade offs between growth of larval structures and the development of juvenile structures. During summer 2025, students and I will have the opportunity to examine the effects of marine heatwaves (MHWs) on larval morphology, swimming speeds, and juvenile development. Data collected will be analyzed using ImageJ and R software and presented at one or more international conferences. The long-term goal of this project is a better understanding of the potential effects of MHWs on the bipinnaria and brachiolaria larval stages of sea stars in the Pacific Northwest. These results will provide insights into the role these larvae play in the recovery of adult populations devastated by SSWD in recent years.
Experimental aquaculture of eelgrass grazers for estuarine restoration
Dr. Sandy Wyllie-Echeverria, Friday Harbor Laboratory, University of Washington
Richelle Tanner, Environmental Science & Policy Program, Chapman University
Seagrass beds are some of the most valuable ecosystems worldwide, providing nursery habitat for fishes and invertebrates, sequestering carbon, and protecting shorelines from coastal erosion. However, they are at disproportionate risk from the effects of coastal urbanization, particularly in ports and areas of high agricultural and industrial runoff. Phyllaplysia taylori, the eelgrass sea hare, is the most efficient grazer of epiphytes on Zostera marina, clearing blades for photosynthesis and growth. During restoration, these sea hares’ vacuuming ability could make the difference for an eelgrass bed struggling to take root under intense epiphyte growth due to urban or agricultural eutrophication. There are strikingly different phenotypes of P. taylori in northern and southern estuaries – they are larger and live longer in the north. If the thinner eelgrass phenotype from Southern California is used for northern eelgrass restoration, it will become necessary to transplant the smaller, Southern California sea hares alongside those eelgrass transplants. This project will be a collaboration with Chapman University, in which we will undertake a field and lab investigation into the environmental drivers of the size and generation time traits to bolster the resilience of eelgrass restoration efforts across the coast.
Biotic and abiotic factors influencing kelp bed communities in the Salish Sea
Dr. Katie Dobkowski, Everett Community College and Friday Harbor Labs
I study marine community ecology and especially focus on foundation species such as bull kelp (Nereocystis luetkeana). Nereocystis is an annual kelp species that provides the bulk of the complex three-dimensional habitat space in rocky subtidal habitats of the San Juan Islands and elsewhere in Washington State. There are a variety of potential summer projects that focus on investigating biotic and abiotic factors influencing bull kelp across their complicated life history using a combination of lab and field work. Topics of interest include investigating:
- green sea urchin (Strongylocentrotus droebachiensis), red sea urchin (Mesocentrotus franciscanus), Northern kelp crab (Pugettia producta), and graceful kelp crab (P. gracilis) feeding preferences (in the lab at ambient and elevated temperatures) and distribution and abundance (in the field; intertidal, snorkel or SCUBA surveys)
- influence of competition from invasive wireweed (Sargassum muticum) on nearshore habitats and species, including bull kelp (Nereocystis luetkeana)
- effects of changing ocean conditions, such as temperature, on invertebrate feeding rates (urchins, kelp crabs)
Subtidal data collection using snorkeling methods is possible, but diving OR snorkeling is NOT a requirement for any of these projects, very successful field data collection can happen on the shore or from boats as well! A better understanding of the dynamics and interactions of N. luetkeana beds in the Salish Sea is crucial not only because they create valuable habitat for economically and ecologically important species, but also to inform management decisions and restoration efforts in a changing ocean.
Animal Behavior in Marine and Terrestrial Environments
Dr. Amy Cook, The Evergreen State College
 One of the greatest challenges animal behaviorists face is observing their subjects in the wild. However, San Juan Island offers opportunities to make detailed observations of the behavior of a variety of animals in the field with relative ease. My mentee will learn the techniques of studying behavior in the field including how to describe behaviors, sampling and recording methodologies, and data collection and analysis. Once they are comfortable with the methodology, my mentee can apply the field behavior techniques they learned to one of the following projects –breeding and social behavior in Pigeon Guillemots or the behavior of juvenile tidepool sculpins (Oligocottus maculotus) in intertidal pools. Animal behavior intersects with conservation biology, ecology, and evolutionary biology and we will discuss these connections in regular research seminars. On San Juan Island, the interaction between human behavior and the behavior of other animals is an important factor in many of our study systems. This project will benefit mentees interested in pursuing careers or advanced study in animal behavior, field ecology, or conservation biology.
One of the greatest challenges animal behaviorists face is observing their subjects in the wild. However, San Juan Island offers opportunities to make detailed observations of the behavior of a variety of animals in the field with relative ease. My mentee will learn the techniques of studying behavior in the field including how to describe behaviors, sampling and recording methodologies, and data collection and analysis. Once they are comfortable with the methodology, my mentee can apply the field behavior techniques they learned to one of the following projects –breeding and social behavior in Pigeon Guillemots or the behavior of juvenile tidepool sculpins (Oligocottus maculotus) in intertidal pools. Animal behavior intersects with conservation biology, ecology, and evolutionary biology and we will discuss these connections in regular research seminars. On San Juan Island, the interaction between human behavior and the behavior of other animals is an important factor in many of our study systems. This project will benefit mentees interested in pursuing careers or advanced study in animal behavior, field ecology, or conservation biology.
Comparing Fish Gut Microbiomes and their Role in Digestion Across the Salish Sea Subtidal and Intertidal Habitats
Dr. Joseph Heras, CSU San Bernardino
The aim of this project is to investigate the gut microbiomes of multiple marine fishes from the Salish Sea by focusing on several factors (e.g., location, diet, taxonomy) and their implications for fish health and potential applications in aquaculture. The gut microbiome, a complex community of microorganisms, plays a critical role in fish digestion, nutrient absorption, and immune function. Student outcomes for this project will include gaining a better understanding of gut microbiomes across different taxa, diets, and habitats. The diverse marine fish families from the Salish Sea that we aim to include in our study are as follows: Cottidae (sculpins), Embiotocidae (surfperches), Gobiidae (gobies), Stichaeidae (pricklebacks), Clupeidae (herrings), Opistognathidae (jawfishes), Liparidae (snailfishes), Pholidae (gunnels), and Gadidae (cod). We will collect species from different locations along San Juan Island and focus on intertidal and subtidal habitats. Intertidal species will be gathered during low tide, while subtidal species will be captured using a seine net during high tide. During the summer internship, the mentee will have the opportunity to gain experience in fieldwork (collecting intertidal and subtidal fishes), rearing live fishes, and conducting gut microbiome extractions required to analyze microbial abundance and diversity. In addition, we will use various bioinformatics tools, including statistical programs such as R, to analyze all digestive physiology and gut microbiome data. The ideal candidate should be interested in intertidal and subtidal fieldwork, maintaining live fishes in the lab, genomics, and bioinformatics. A student with experience in any of these areas is preferred but not required.
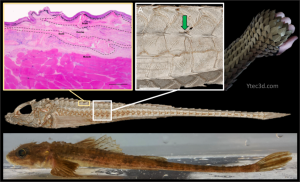
The Functional Morphology of the Armor-Skin Composite in Poacher fishes
Dr. Karly Cohen, UW-Friday Harbor Labs/Biological Sciences-CSU Fullerton
Dr. Cassandra Donatelli, UW Tacoma School of Engineering and Biology
Poachers, belonging to the family Agonidae, are marine fishes that inhabit cold, coastal waters, primarily in the northern Pacific Ocean. These bottom-dwelling fish are renowned for their striking appearance and unique adaptations: their bodies are covered in bony armor plates that protect them from predators while still allowing for mobility. This project will delve into the interplay between their armored scales and movement.
Poacher scales overlap to varying degrees, with gaps along the body filled with unknown connective tissues. These gaps likely play a crucial role in enabling the fish’s movement while providing protection and may also store energy, helping the fish make quick escapes when threatened by predators. As an REU you will investigate these spaces and their functional significance, focusing on two main aspects:
- Fish Movement: How do the spaces between the scales contribute to the fish’s ability to swim and maneuver efficiently?
- Scale Interactions: What role do these spaces play in allowing flexibility and energy absorption during external impacts, such as predator attacks or collisions with obstacles?
Research Outline
- Fishing for Knowledge (Literally!)
- We’ll begin by heading out to the field for fishing expeditions to collect poacher fish specimens from their natural habitat. If you enjoy fishing, this will be an exciting and hands-on way to start our research!
- Cellular Morphology and Histology
- Back in the lab, we’ll analyze the microstructures of the scales and the soft tissues in the spaces between them. Using advanced histological techniques, you’ll learn how to prepare and examine tissue samples, interpret cellular morphology, and understand the biological properties that allow for flexibility and durability.
- Advanced Visualization Techniques
- To understand the role of the connective tissues between individual armor plates, we will use materials analysis coupled with Digital Image Correlation – DIC. DIC will let us track how individual plates move when the fish bends and twists.
- Bioinspired Modeling and Testing
- Armed with insights from histology and material testing, we’ll create multi-material 3D-printed models of poacher fish armor. These models will allow us to quantify the behavior of a scale-skin composite. We will use our results to explore applications for human use, such as protective armor, flexible robotics, or shock-absorbing materials.
An interest in fishes, morphology, and interdisciplinary research is essential, as this is not an ecology-focused project. You will be involved in fieldwork, computer-based analysis, and histology preparation using chemicals. You will receive thorough training in proper animal care and chemical safety.
We encourage our REU students to manage their time effectively, take full advantage of their experience at Friday Harbor Labs, and immerse themselves in the unique opportunities here. We love seeing students out on the boats, engaging with the research community, and collaborating with fellow apprentices.
Characterizing sensory and swimming systems of marine invertebrate larvae to enable conservation and understand animal evolution
Dr. Eric Edsinger, Whitney Laboratory for Marine Bioscience, University of Florida
My research integrates functional genomics, neuroscience, and biodiversity. Its focus is on sensory biology and cilia-based swimming of marine invertebrate larvae, framed in greater contexts of environmental change and conservation, in addition to early origins of the brain and behavior in animals. One example is leveraging genetic tools in neuroscience to understand potential impacts of marine heatwave conditions on settlement and metamorphosis of marine invertebrate larvae in lab, as environment-driven changes in a larva’s decision to settle or not and its subsequent success in metamorphosis or not, can impact the distribution and survivorship of benthic marine invertebrates, while a greater understanding could enhance modeling and management in conservation. Another is again leveraging genetic tools in neuroscience but now to characterize use of “neurotransmitters” found in human but also utilized in sensory systems and swimming behaviors of marine invertebrate larvae lacking or prior to development of a nervous system (meaning, neural genetic components underlying human behavior are potentially utilized by aneural or preneural marine invertebrate larvae in their behavior), with implications for the origins of our brain and early evolution of multicellularity in animals.
REU summer research will include:
- field collection, spawning, and lab culturing of marine invertebrates and their embryos and larvae
- injection of genetic tools into 1-cell embryos for microscopy assays of genetic tool read outs, anatomy, and behavior at later larval stages
- pharmacological assays of neurotransmitters and ion channel agonists/antagonists and/or environmental change assays (temperature and/or viscosity)
- confocal microscopy imaging of live and fixed embryos and larvae, including use of fluorescent dyes and immunohistochemical-labeling of cilia and the nervous system, and/or
- bioinformatic genetic tool design and phylogenomic analyses of gene family evolution.
Possible animal groups include cnidarian jellyfish and genetic lines of the lab model Hydra, echinoderm sand dollars, mollusc cephalopods (if permitting allows), nudibranchs, and snails, and/or urochordate sea squirts. Experience in Unix, Python, and/or R is preferred but not required. Balance is greatly encouraged but work is intense and may include odd-hours due to developmental timing of embryos and larvae – so an openness to a shifting schedule based on animals and tides is ideal. The below image is of the nudibranch mollusc Melibe and the sea squirt urochordate Corella.
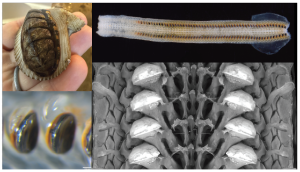
Iron teeth and nothing to chew yet: tooth development in larval chitons
Dr. Rebecca Varney, University of Nebraska-Lincoln
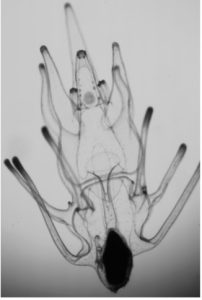
Chitons are marine molluscs that make iron teeth. Iron is toxic because it catalyzes the production of free radicals. Yet chitons grow iron teeth constantly across their lifespans, creating a new row of teeth approximately every 3 days. This constant iron use means chitons have 20x more iron circulating in their bodies than any other animal. So how do chitons use so much iron without dying? One key to this puzzle is that chiton larvae don’t make their teeth from iron at first. They make teeth on their radula (an organ like a tongue) but don’t biomineralize with iron until they are almost a month old. What’s happening in the larval teeth? What are those teeth made of, and how do chitons ramp up iron use? Friday Harbor Labs is situated in the cradle of chiton diversity, so samples abound, but no timecourse of radula development exists yet for chitons. We will work together to spawn chitons, fertilize eggs, and generate preserved specimens of larval development. You will learn marine animal husbandry and spawning, larval culture, light microscopy, SEM, and potentially some antibody labelling and confocal if you are interested. I will be teaching Invertebrate Zoology, so you will have access to flow tables and accompany us on field trips to collect chitons. Let’s find out what baby chitons are up to!
Figure: the iron teeth of chitons. Clockwise from top left, a chiton at FHL, a radula, an SEM image of chiton teeth, and light microscopy showing the shiny, black iron.
Uncovering passive dynamics of batoids
Bart Boom, PhD Candidate, Department of Aeronautics and Astronautics, University of Washington
Batoids have complex undulatory patterns when propulsing themself. Their complex fin structure with +-100 finrays and thousands of radials connects to tendinous muscle sheets. The goal of this project is to find out how much of their motion is passively generated. We will experimentally investigate this question by quantifying the motion of batoids at different actuation frequencies using camera tracking. Additionally, we will measure the thrust produced and the flow field using a force transducer and Particle Image Velocimetry. When we have quantified the biological specimens, we will try to emulate it using artificial batoids. The project will include testing in a flume (water tunnel), CT-scanning specimens, Python coding, 3D modeling, and manufacturing techniques like 3D printing, laser cutting, and silicone molding.
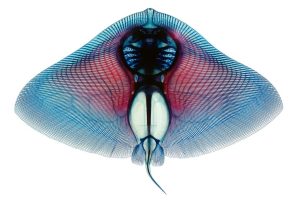
How does seahorse morphology relate to their ability to produce exceptionally rapid feeding strikes?
Roi Holzman, Tel-Aviv University and The Inter-University Institute in Eilat
Seahorses, pipefish, and some of their relatives can generate ultra-fast feeding strikes by storing elastic energy in their tendons and releasing it abruptly. This mechanism enables these fish to complete feeding strikes within a millisecond or two—almost ten times faster than any other fish. While the location of energy storage is understood, the mechanics of power transmission through the head remain unclear. Additionally, the process by which the system is latched and triggered is still a mystery. To address these questions, we will conduct detailed morphological measurements across a variety of fish species within the clade that includes seahorses. We will use CT scans to identify and measure the parts of the skull that transmit motion and power during the strike. These measurements will help parameterize a mechanical model of force transmission and investigate how the diversity in shape and size of these fish influences their feeding capabilities.