 Often our Tide Bites show the wide reach of FHL, through both space (e.g. Dec. 2024 Tide Bite) and time (e.g. July 2024 Tide Bite). The broad and deep connections of the FHL Family are not only professionally rewarding and fun, such as FHL gatherings at the Society for Integrative and Comparative Biology (SICB) meetings each year, but they can also pop up to support current research efforts as described here. Our connections with Northeastern University, through its long-running Three Seas Program (some may remember it as East-West), and the personal linkage of Ken Sebens, enabled Kindall to seize a data-gathering opportunity that wound up providing critical context for her dissertation research. FHL may be on an island, but it’s a well-connected one!
Often our Tide Bites show the wide reach of FHL, through both space (e.g. Dec. 2024 Tide Bite) and time (e.g. July 2024 Tide Bite). The broad and deep connections of the FHL Family are not only professionally rewarding and fun, such as FHL gatherings at the Society for Integrative and Comparative Biology (SICB) meetings each year, but they can also pop up to support current research efforts as described here. Our connections with Northeastern University, through its long-running Three Seas Program (some may remember it as East-West), and the personal linkage of Ken Sebens, enabled Kindall to seize a data-gathering opportunity that wound up providing critical context for her dissertation research. FHL may be on an island, but it’s a well-connected one!
Flume to Field: Measuring Seawater Chemistry Within Mussel Beds from Coast to Coast
by Kindall Murie
In science, sometimes you have to go with the flow — unless you’re a mussel, which has a knack for staying put. While mussels are well-known for anchoring themselves to rocky shores using sturdy byssal threads, their real magic lies in how they shape their environment. By forming dense aggregations, mussels not only provide habitat for over 300 species but also interact with their surroundings in fascinating ways (Suchanek, 1978; Carrington et al., 2009). Flow conditions, in particular, influence how mussels alter their local chemical environment, which in turn may impact their behavior, such as feeding and gaping open. This dynamic relationship between flow, chemistry, and behavior became the focus of my research.
For years, I’ve been fascinated by how organisms alter their chemical surroundings through metabolic processes and how these changes can affect other organisms. Before joining Dr. Emily Carrington’s lab in UW Biology, I studied how kelps modify local chemistry through photosynthesis and respiration (Murie & Bourdeau, 2020). I was keen to continue this work at FHL, and first explored how strong tidal currents in the San Juan Islands limit the ability of Bull Kelp (Nereocystis luetkeana) to change both pH and dissolved oxygen (Murie et al., in prep). I then transitioned from Team Kelp to Team Mussel to ask similar questions about how flow affects the localized microclimates created by mussel aggregations — part of an NSF-funded collaborative project led by Dr. Emily Carrington, Dr. Matt Reidenbach (University of Virginia) and Dr. Mike Nishizaki (Carleton College).
For mussels, metabolic activity drives chemical changes within the interstitial spaces (the small complex spaces between mussels) of an aggregation (George et al., 2022). High-flow environments quickly flush water through the aggregation, minimizing the buildup of metabolites like ammonia (a waste product excreted by mussels), while low-flow conditions allow chemical gradients, such as oxygen depletion, to form (Ninokawa et al., 2020). Flow conditions, however, can also influence mussel behaviors — such as gaping and filter feeding — which are closely linked to their metabolic activity and can, in turn, create feedbacks that alter the local chemistry. Unlike kelps, which lack behavior to regulate metabolic activity, mussels introduced an exciting new aspect for me to explore.
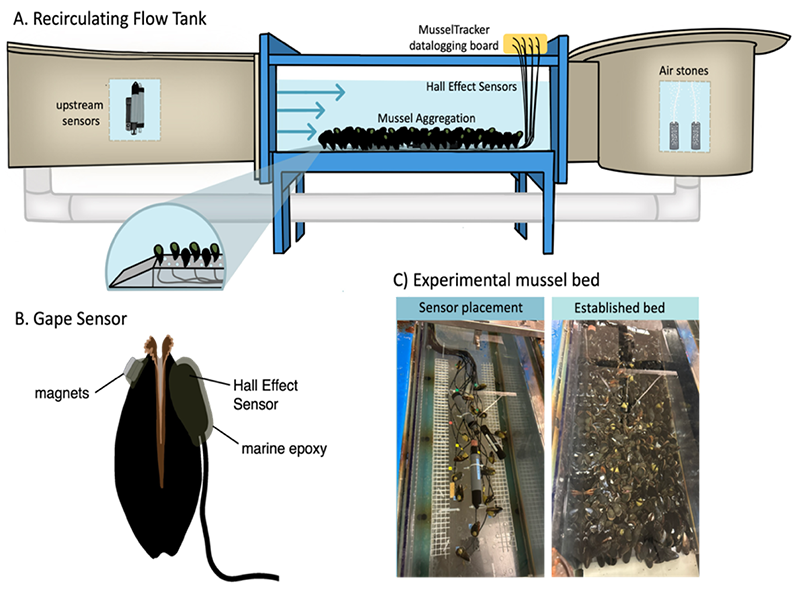
Testing Flow, Gaping, and Chemistry in the Lab
During feeding, mussels gape (open a small gap between their shells) to draw in water, capture food, and expel filtered water. This process could mix the interstitial water and reduce the chemical gradients that form in low flow conditions (hypothesis 1). In other words, mussels might actively “mix themselves out of trouble”. Alternatively, strong chemical gradients might stress the mussels, causing them to close (“clam up”) until more benign conditions return (hypothesis 2). To test these hypotheses, I conducted a lab experiment exposing a single-layer mussel bed to a range of flow speeds while measuring gaping behavior and dissolved oxygen (DO) inside and outside the bed.
Using the large-volume flume in Lab 6 at Friday Harbor Labs, I created a dense mussel aggregation and applied magnetic gape sensors (Miller & Dowd, 2017) to measure when mussels were actively gaping (Figure 1A-C). Oxygen sensors placed between mussels and upstream measured the difference in DO (ΔDO = Ambient – In-bed). For 10 flow speeds ranging from 1–30 cm per second, I monitored both gaping behavior and chemical changes. I also compared responses across three common mussel species in Washington (Mytilus trossulus, M. galloprovincialis, and M. californianus), though I’ll focus on M. trossulus here because the results were similar across species.
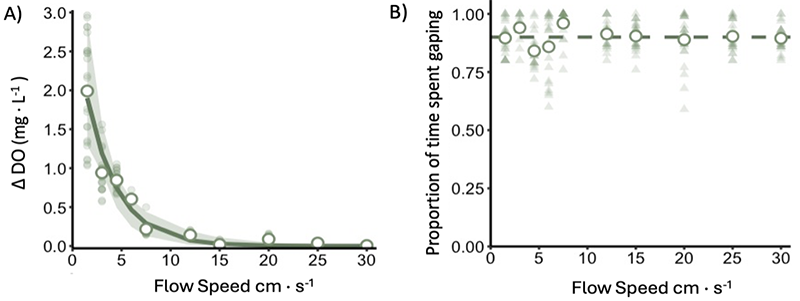
As expected, DO gradients decreased as flow increased, with the largest gradients forming at speeds below 5 cm∙s⁻¹ (Figure 2A). At higher flow speeds, water flushes readily through the mussel aggregation such that there was no difference in oxygen between ambient water and the interstitial zone. In contrast, gaping behavior remained consistent across all flow speeds: M. trossulus spent more than 85% of its time gaping regardless of the stronger DO gradients at low flow speeds (Figure 2B). These findings challenge both of my initial hypotheses! One caveat, however, is the gape sensors may not be the perfect proxy for filtering behavior. It’s still possible that mussels can modulate their pumping rate while maintaining a consistent gape, thus changes in DO altered by mussels filtering is still possible. Further work is being conducted by Dr. Emily Carrington and undergraduate Kathryn Farabaugh to look at the relationship between gape and filtering velocity.
From Lab to the Field: A Rare Opportunity
While lab experiments are valuable for allowing control of environmental variables, they cannot fully replicate the complexity of natural mussel beds. The maximum gradient in dissolved oxygen I observed in the lab was 3.0 mg∙L⁻¹, not enough to explain the low oxygen levels reported by George et al. (2022) at a mussel farm. I suspected the difference was the thickness of the mussel aggregation: my experiment was monolayer while mussels in farms and on many natural shores are multilayer. While considering local options for field observations, such as on the Washington outer coast, an exciting opportunity arose when Dr. Ken Sebens returned from Northeastern University’s Marine Science Center (NU MSC) in Nahant, Massachusetts and reported an enormous recruitment of bay mussels (M. edulis) covering the seafloor at his long-term study sites. Recognizing this as a unique chance to measure how multilayer beds could alter the local chemical environment, Emily and I hopped on a plane to see it for ourselves.
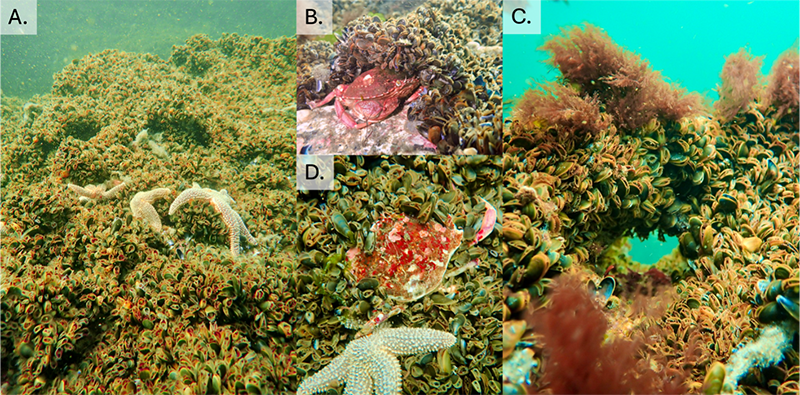
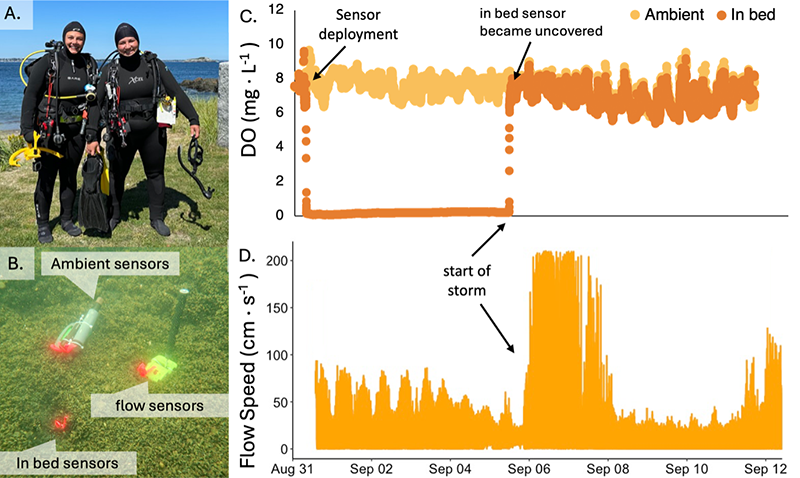
Holy mussels, Ken wasn’t lying! I had never seen so many mussels in my life. The mussel beds swallowed the reef, leaving no bare rock in sight (Figure 3 and guided video). Sea stars and crabs were everywhere, actively feeding but also creating tunnels and caves throughout the bed (Figure 3A-C). I even saw crabs that looked like they had been literally swallowed up by their food, with mussels growing on top of their carapace (Figure 3D). With logistical help from Dr. Brian Helmuth’s lab at MSC and divers Angela Jones and Breck McCollum, we deployed oxygen sensors inside and outside of these mussel beds, along with bulk flow meters above the mussel beds.
The incredible density and depth of these mussel beds had a huge impact on oxygen levels inside the mussel bed. Once the DO sensor was placed inside the bed, we saw DO immediately drop to 0 mg·L-1, indicating that the reef below the mussels was anoxic (Figure 4D). This was visually obvious from the black cloud of anoxic sediment that spilled out from between the mussels when putting the sensor in. The low DO lasted only a few days, however, because a large storm that rolled in (denoted by the flow sensor measuring velocities well above 100 cm∙s⁻¹) likely uncovered the sensor (which is how Angela found it upon retrieval) and returned oxygen levels to ambient – matching the sensor that was placed outside the mussel bed (Figure 4C). Overall, this enormous recruitment of bay mussels shows us just how influential mussels can be in changing their chemical environment. The opportunity to measure such dramatic, mussel-driven changes in the field was made possible through the global connections and resources cultivated by Friday Harbor Labs. This unique blend of science and camaraderie is what makes FHL truly special.
Piecing the Puzzle Together
The lab and field studies together provide valuable insights into how mussels alter their local chemical environment. In the lab, we showed that chemical changes are flow-dependent, but that gaping behavior remains constant even at low flows. In the field, we observed the full extent of mussel-driven anoxia in dense, multilayered aggregations. The next step is to connect these findings: under extreme chemical changes, like those in Nahant, does gaping behavior change?
Understanding the role of mussels as habitat-forming species requires linking behavior, environmental conditions, and chemistry across scales. These studies highlight how mussels not only create habitat for other organisms but also profoundly influence the chemical environment within their aggregations. This work is a reminder of how much there is to discover when we look closely at the interactions between organisms and their surroundings — especially when mussels, against all odds, go with the flow.
This project was supported by the National Science Foundation (GRF to KM and OCE-2050273 to EC), UW Student Technology Fee, and numerous fellowships and scholarships from UW Biology (Friday Harbor Laboratories award, Hoag Award, John S. Edwards Award, Margo & Tom Wyckoff Award) and UW Friday Harbor Labs (Patricia L. Dudley Endowment, Frederic H. and Kristin C. Nichols Endowed Graduate Fellowship fund, Emily Carrington Endowed Student Travel Support, FHL Research Fellowship Endowment, and Brooks and Suzanne Ragen FHL Endowed Scholarship). I thank FHL Director Megan Dethier and FHL staff for logistical support, Dr. Luke Miller for help with the gape sensors, the Carrington, Reidenbach and Nishizaki labs for expertise and advice, and the dive safety officers at both UW and NU.
References
Carrington E., Moeser G.M., Dimond J., Mello J.J., and M.L. Boller. 2009. Seasonal disturbance to mussel beds: field test of a mechanistic model predicting wave dislodgment. Limnology and Oceanography, 54(3), 978-986.
George M.N., O’Donnell M.J., Concodello M., and E. Carrington. 2022. Mussels repair shell damage despite limitations imposed by ocean acidification. Journal of Marine Science and Engineering, 10(3), 359.
Miller L.P. and W.W. Dowd. 2017. Multimodal in situ datalogging quantifies inter-individual variation in thermal experience and persistent origin effects on gaping behavior among intertidal mussels (Mytilus californianus). Journal of Experimental Biology, 220(22), 4305-4319.
Murie K.A., and P.E. Bourdeau. 2020. Fragmented kelp forest canopies retain their ability to alter local seawater chemistry. Scientific reports, 10(1), 11939.
Ninokawa A., Takeshita Y., Jellison B.M., Jurgens L.J., and B. Gaylord. 2020. Biological modification of seawater chemistry by an ecosystem engineer, the California mussel, Mytilus californianus. Limnology and Oceanography, 65(1), 157-172.
Suchanek T.H. 1978. The ecology of Mytilus edulis L. in exposed rocky intertidal communities. Journal of Experimental Marine Biology and Ecology, 31(1), 105-120.
Tide Bites is a monthly email with the latest news and stories about Friday Harbor Labs. Want more? Subscribe to Tide Bites or browse the archives.