JULY 19 – AUG 8, 2026
Functional Biodiversity
Genomics, Genetics, Imaging & AI in Aquatic Invertebrates
Three weeks. Three phyla. One breakthrough technology.
Work hands-on with cnidarians, echinoderms, and cephalopods—from collection and culturing to genetic modification and AI-driven analysis. You’ll work with two Nobel Prize species: the GFP jellyfish Aequorea victoria and the squid giant axon action potential species Doryteuthis pealeii. And you will help pioneer RNA-sensing technology in marine invertebrates for the first time!
Click Here To Apply to a Summer 2026 Training Workshop
At a Glance
Dates: July 19 – August 8, 2026
Location: Friday Harbor Labs, San Juan Island, WA
Format: Intensive, hands-on, small groups
For: Grad students, postdocs, early-career researchers
Bonus: Co-authorship opportunity on RNA-sensing paper
What You’ll Do
Design. Culture. Modify. Image. Discover.
Each week focuses on a different animal group. You’ll work with established genetic lines of lab models, apply cutting-edge tools to local species—often for the first time—and test new-to-science RNA-sensing constructs. Along the way, you’ll explore the origins of brains and nervous systems and discover new models for aging resistance hidden in biodiversity.
Genomics & AI
Build phylogenomic pipelines across cnidarians, echinoderms, and cephalopods. Explore whole-organism connectomics. Model behavior using whole-organism, whole-nervous-system dynamics.
Genetic Tools
Design and test RNA-sensing constructs, antibodies, and biosensors. Perform microinjection. Execute shRNA knockdowns and CRISPR knockouts.
Imaging
From phone-based documentation to light-sheet microscopy and microCT. Live and fixed samples, embryos to adults.
Culture & Husbandry
Spawning, fertilization, embryo culture. Genetic line maintenance. Cell and tissue culture establishment.
The RNA-Sensing Breakthrough
RNA-sensing is a new technology that targets genetic tools to specific cell types based on native gene expression. Classically, targeting requires intensive development of specific promoters for each cell type—a slow, labor-intensive process.
Here’s the elegance of RNA-sensing: it requires only a single ubiquitous promoter. No painstaking promoter development for each target. Instead, you design a short targeting sequence (200–300 bp) from a cell-type-specific transcript. A built-in stop codon blocks expression unless the matching native transcript is present, which corrects the stop and activates your payload.
Simple. Scalable. And now ready for marine systems.
RNA-sensing is already revolutionizing mouse and human research—and holds special promise for non-model species in biodiversity, where research communities and resources are often limited.
RNA-sensing has never been used in marine animals. Until now.
Workshop participants will test new RNA-sensing constructs across cnidarians, echinoderms, and cephalopods—pioneering this technology in non-model organisms. If successful, participants will co-author a subsequent research paper.
Cell Lines For Scalable Functional Assays
Functional research in aquatic invertebrates typically depends on limited numbers of animals. This constrains throughput and makes large-scale screening impractical.
Cell lines change that equation.
By establishing stable cell cultures from embryonic tissues, researchers can test hundreds of genetic constructs, optimize conditions rapidly, and develop standardized functional assays—approaches routine in mammalian systems but rare in marine biology. Participants will learn to establish embryonic cell lines in sea urchins and potentially other species, bridging traditional approaches with scalable, assay-based methods.
Why Functional Biodiversity Matters
Evolution has tested and optimized for billions of years in its engineering of Life. Every genome is a library of tested solutions—protein structures, neural architectures, aging resistance mechanisms. Genomic sequencing is exploding and databases are growing exponentially. But data without function is just a parts list.
This workshop bridges that gap.
You’ll combine cutting-edge genomics, genetic manipulation, advanced imaging, and AI to decode how life actually works—venturing beyond classic genetic models and into the Tree of Life. The insights won’t just advance marine biology. They’ll illuminate principles and components for human health, biotechnology, and conservation.
Evolution has done the R&D. Let’s explore its handiwork.
The Animals
Cnidaria: Jellyfish & Polyps
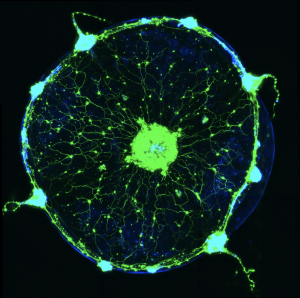
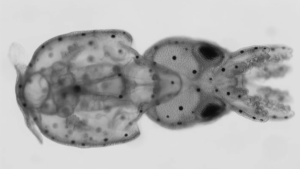
Hydra vulgaris — Polyp – GCaMP genetic line
Clytia hemisphaerica — Jellyfish – RCaMP genetic line
Clytia gregaria + Aequorea victoria — Jellyfishes – field-collected [GFP NOBEL PRIZE]
Echinodermata: Sea Stars, Sea Urchins & Sand Dollars
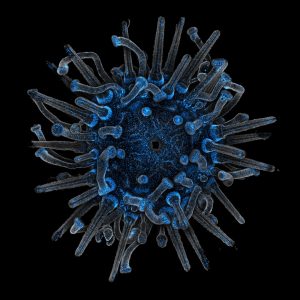
Lytechinus pictus — Sea urchin – TBD genetic line
Dendraster excentricus — Sand dollar – field-collected
Cephalopoda: Squid, Cuttlefish & Octopus

Sepia bandensis — Cuttlefish – TBD genetic line
Doryteuthis pealeii — Squid, field-collected CRISPR [ACTION POTENTIAL NOBEL PRIZE]
Doryteuthis opalescens + Octopus rubescens — Squid and Octopus – field-collected)
Training
Genetic Tools
- Fluorescent protein reporters
- RNA-sensing (pioneering work!)
- Vitellogenin transfection
- shRNA knockdowns
- CRISPR knockouts (TBD cilia genes)
- Biosensors – Calcium / Serotonin
- Controllers (TBD optogenetic PdCO)
Imaging
- Phone-based documentation
- Live-tracking imaging
- Fluorescence microscopy
- Confocal microscopy
- Light-sheet microscopy
- MicroCT microscopy
Field & Lab
- Salish Sea species collection
- Embryo culture & maintenance
- Genetic line husbandry
- Gonad tissue culture
- Embryonic cell lines
- Neurotransmitter pharmacology
Genomics & AI
- Phylogenomic pipeline design with AI
- Species & gene tree construction
- HMM protein annotation
- Orthogroup genome clustering
- Origin-conservation-loss mapping
- AI data exploration
AI/ML/Modeling
- Behavior quantification
- Connectomics
- Whole-organism, whole-nervous-system modeling
- Computational protein engineering
The Instructors
Adrienne Fairhall | University of Washington — Theoretical Neuroscience
Amro Hamdoun | UC San Diego — Echinoderm genetic lines (Remote)
Andrea Bodnar | Gloucester Marine Genomics Institute — Cell lines and Aging
Brandon Weissbourd | MIT — Cnidarian neuroscience
Connor Gibbons | Columbia University — Cephalopod culturing
Eric Edsinger | University of Florida — Animal origins and evolution
Fabian Voigt | Harvard University (Engert Lab) — Live tracking microscopy
Isabella King | University of New Mexico (UF Edsinger Lab) — Sand dollar behavior
Jason Hodin | University of Washington — Echinoderm biology & conservation
Jason Qian | University of Washington (Baker Lab) — Protein engineering
Jeremy Koob | University of Washington (Baker Lab) — Protein engineering
Jessica Stock | Marine Biological Laboratory (Albertin Lab) — Cephalopod Neuroscience
Josh Huang | Duke University — RNA-sensing technology
Shulin Zhang | Stanford University (Hernandez-Nunez Lab) — Hydra connectomics
Sijie Xia | Boster Bio — Antibody design (Commercial)
Tessa Montague | Columbia University (Axel Lab) — Cephalopod neuroscience
Program Structure
Duration: Three weeks, intensive hands-on format
Location: University of Washington’s Friday Harbor Laboratories, San Juan Island, Washington—a premier marine station with direct access to the incredibly rich biodiversity of the Salish Sea
Daily schedule: Laboratory work with embryos, larvae, adults, and/or cell and tissue cultures; expert-led instruction; data analysis sessions; field collection; collaborative research
Small group format: Direct mentoring from faculty instructors, working in teams on shared research goals
Who Should Apply
This workshop is designed for graduate students, postdocs, and early-career researchers who want to:
- Add genetic tools and cell-based assays to their research toolkit
- Work across species diversity, not just model organisms
- Combine molecular, cellular, and computational approaches
- Scale up functional genomics in non-model systems
- Apply cutting-edge methods to their own research questions
Prerequisites: Graduate-level training in biology, bioinformatics, or related STEM fields. Prior experience with molecular biology, cell culture, or microscopy is helpful but not required—the training is high-level, and you’ll learn hands-on.
Ready to Pioneer?
Applications are open. Join us at one of the world’s premier marine stations to push the boundaries of functional genomics in the ocean’s most fascinating invertebrates.
Application Materials Must Include:
- PDF of your Statement of Purpose: write 500 words or more indicating your (1) interest in the chosen training workshop, (2) how the workshop will influence your career path. (3) what aspects of the workshop you are most interested in, (4) a statement of current research or research interests
- PDF of your CV
- Name and contact information for one or more references
- PDF of your unofficial transcript if applicant is an undergraduate or not yet in graduate school
Click Here To Apply to a Summer 2026 Training Workshop
Workshop design is ongoing. Details may be updated through Spring 2026.
Friday Harbor Laboratories · University of Washington