 Often, scientific advances are made when new methods are used to tackle old problems, like using modern genomics methods to study cancers. This essay by Allie Tissot takes the opposite approach, using old methods (tried and true larval culturing techniques) to study new problems: impacts of modern aquatic contaminants on organisms. I love the fact that Allie is at least the third “generation” of scientists to do larval culture studies at FHL, constructing a “Strathmann stirrer” to raise larvae and building on knowledge of seastar culture gained over recent years by Jason Hodin, himself a Richard Strathmann protégé. Scientific tradition can be a powerful tool!
Often, scientific advances are made when new methods are used to tackle old problems, like using modern genomics methods to study cancers. This essay by Allie Tissot takes the opposite approach, using old methods (tried and true larval culturing techniques) to study new problems: impacts of modern aquatic contaminants on organisms. I love the fact that Allie is at least the third “generation” of scientists to do larval culture studies at FHL, constructing a “Strathmann stirrer” to raise larvae and building on knowledge of seastar culture gained over recent years by Jason Hodin, himself a Richard Strathmann protégé. Scientific tradition can be a powerful tool!
The Fellowship of the Larvae
by Allie Tissot
Allie is a PhD student at Portland State University focusing on the effects of pesticides as multiple stressors on aquatic invertebrate physiology. She earned her B.S. in Environmental Science with an emphasis in Marine Ecology at Western Washington University and has since contributed to various projects. Before starting graduate school, she worked for three years as a lab and field technician in central Chile, assisting on numerous projects including larval connectivity among marine protected areas and the effects of hypoxia on maternal investment in kelp crabs. In her dissertation work, she is currently conducting a study to quantify field detections of organic contaminants before and after the spring industrial spray season in collaboration with the Confederated Tribes of the Coos, Lower Umpqua, and Siuslaw Indians (CTCLUSI) and the United States Geological Survey (USGS). Her work at FHL was funded by the Charles Lambert Memorial.
Working at FHL was something I had been dreaming of since studying at WWU. In 2014 during one of my invertebrate zoology courses, we traveled to San Juan Island for an invertebrate scavenger hunt. Our professor and my advisor, Brian Bingham, mentioned that we were close to a famous marine research institution called Friday Harbor Laboratories. From that moment on, I hoped to return and take a course someday. I never imagined that I’d actually become a researcher and conduct my own experiment there.

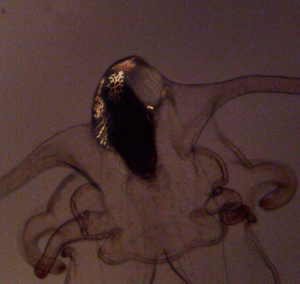
Near the end of my undergraduate degree, sea star wasting disease (SSWD) spread along the coast. Where there were once blankets of sea stars at the coastal parks in Bellingham, you were suddenly lucky to see one or two. It was a prevalent event during my final undergraduate years, and stayed in the back of my mind as I began my career. My work in Chile led me to cultivating kelp crab larvae, and I fell in love with larval development. Both of these newfound passions became part of my past as I began to work with pesticides and adult invertebrates in graduate school. One day my advisor, Elise Granek, reached out with an exciting fellowship opportunity she thought might interest me. She told me she had spoken with Jason Hodin at FHL about wanting to study how water contaminants may affect the larvae of Pycnopodia helianthoides (Sunflower stars) and asked if I’d lead a project. As Pycnopodia were now endangered due to impacts from SSWD, the Hodin lab had been culturing their larvae to further understand their life history and support future captive rearing efforts for recovery of the species. They had so far raised larvae from two spawning events to their juvenile stage and were conducting experiments to look at temperature preferences. Jason was expecting a spawning event for their third cycle of larvae, and I would be able to culture some of them with my experiment. I was so excited I actually yelled. This project gave me the chance to link my love of toxicology with my fascination with cultivating larvae. What’s more, I’d be studying a gorgeous sea star that I’d only ever seen one time in the intertidal a decade ago (Figure 1). As nature would have it, the adult stars unexpectedly spawned three weeks before I was supposed to travel to FHL, meaning I wouldn’t make it in time to start my experiment and the project was postponed for another year. However, the extra time only expanded my excitement and before I knew it, I was loading up my car and driving up to San Juan Island to work with the fourth round of larvae.
Deciding which contaminants to work with was challenging because in the environment there’s a massive array of pesticides, pharmaceuticals, personal care products and microplastic types, all mingling and interacting together to create a cocktail of anthropogenic waste. After much deliberation, we narrowed it down to two contaminants: imidacloprid and polyester. Imidacloprid belongs to a class of insecticides called neonicotinoids, which were banned from the European Union in 2018 for their toxicity to pollinators. They are, however, still widely used in the U.S. and frequently detected in aquatic environments. Polyester microfibers are among the most commonly detected microfiber types in the environment, making them an essential contaminant to consider. These synthetic fibers shed from our clothing — as we both wear and wash them — and enter the environment.

Along with Jason and my PhD committee, I designed a six-week study using environmentally relevant concentrations of each contaminant, which would span the entire larval development period up until settlement. Focusing on chronic exposure is more environmentally realistic, as aquatic organisms are often exposed to contaminants at these lower concentrations over a longer, more consistent period of time. Given that this was my first time ever working with Pycnopodia larvae – which Jason referred to as the “black diamond” of echinoderm larval cultivation (i.e., much more difficult to raise in the lab than other echinoderms, such as sea stars, urchins, or sand dollars) – and the implementation of contaminant stressors, we weren’t sure how long the larvae would survive. Jason’s technicians, Augie and Fiona, gave me a crash course in Pycnopodia cultivation, teaching me everything and guiding me in set up of the laboratory (Figure 2). After two weeks of (literally) running around and creating a whole new larval lab, we were ready to start the experiment.

The larvae were raised in mason jars kept under a device called a Strathmann stirrer (invented by Richard Strathmann), which employs the use of paddles to gently create currents so that the larvae are able to constantly move (Figure 3). Their general care included a water change and feeding every other day, which involves taking each jar and filtering out the larvae in order to clean the jar and give them fresh water. After filtering, we were able to take a look at the larvae under a dissecting microscope to make general observations, and each week we would collect samples. On sample days, the total number of larvae in each jar was counted and we would then randomly select 25 larvae to photograph for measurements. For the sake of time, I would arrange 10 larvae on a raised slide and take live photos using a camera attached to a compound microscope, while the other 15 were fixed for later photographing.
The experiment proved to be an incredible and intense experience: I learned how to cultivate these larvae while conducting a multiple-stressor experiment. It was full of all sorts of speed bumps, from broken glassware to a jar of larvae accidentally poured down the sink (my bad) to sampling days that went into the late hours of the evening. Despite the challenges, we achieved something I wasn’t sure was possible in the beginning. We made it through the six weeks to juvenile settlement, and it was honestly one of the most fascinating and rewarding experiences of my career so far. I was reminded of why I love larval biology so much and I was able to work with a species that few people have gotten to study in recent years.
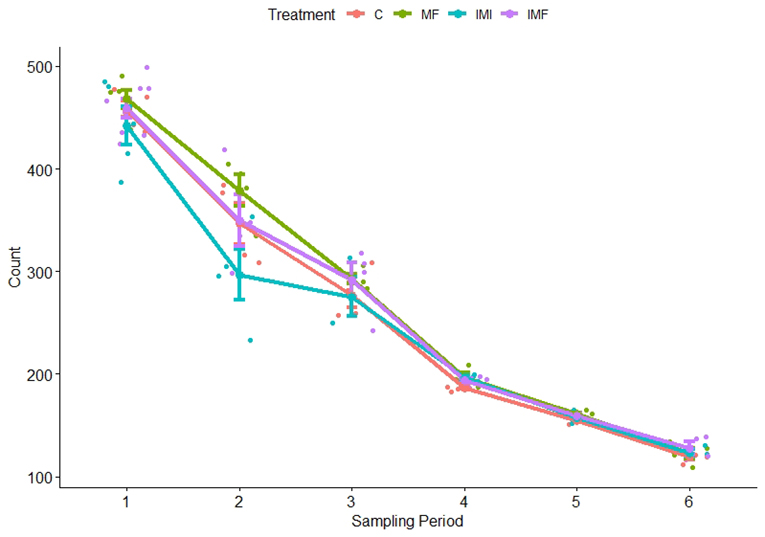
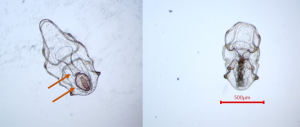
I just completed these experiments in March and am still analyzing the data, but initial observations indicate there are impacts of toxicity from imidacloprid as well as effects on settlement from microfiber exposure. The total number of larvae being raised is expected to decline due to natural mortality as larval development is extremely challenging. Additionally, I was removing larvae for my samples and reducing the density at two dates in order to avoid overcrowding as they grew bigger. However, in week two there was a clear difference in survival between the imidacloprid treatment and the others (Figure 4). It was at this time that we observed larvae with contorted digestive systems (Figure 5), but only in the imidacloprid treatment. Coupled with the decrease in larval counts, this suggests that imidacloprid is toxic to these larvae during gut development. The most remarkable observation in the microfiber treatments was made near the end of development. Once the larvae were in their late brachiolaria phase and getting ready to settle, we noticed them attempting to settle on the fibers themselves. They were beginning to metamorphose and were incredibly difficult to remove. This had been observed in previous cultivations done by the Hodin lab when unintentional microfiber contamination was present. A larva attempting to settle on a microfiber in its natural environment is unlikely to survive juvenile metamorphosis. Not only are the microfibers not large enough to provide habitat for a juvenile sea star, but they are likely being encountered in the water column and farther away from the resources the stars need to continue to grow.
Moving forward, I am in the process of measuring other parameters that might have been affected by the contaminants, including changes in larval length and key developmental features such as brachiolar structures from samples at weeks 4-6 (Figure 6). Studying these contaminants at environmentally relevant concentrations over chronic time scales is key to understanding what we might begin to observe as organisms experience increasing levels of contaminant exposure in their natural habitats. While not directly correlated with SSWD, this study and others like it are crucial to understanding why these animals struggle to recover from pathogens or changes in oceanic conditions that are progressing with climate change.
I am immensely grateful for the funding provided by the Charles Lambert Endowment and to the Hodin lab for not only providing this opportunity, but also in guiding me and helping me conduct the experiment. I also want to acknowledge everyone at FHL: techs, students, researchers, maintenance, and office staff. I met so many delightful and wonderful people and you all made this experience memorable. Thank you.
Resources
Granek E.F., Traylor S.D., Tissot A.G., Hurst P.T., Wood R.S., and S.M. Brander. 2022. Clothes Encounters of the Microfibre Kind: The effects of natural and synthetic textiles on organisms. Polluting Textiles, 63-99. Routledge.
Hodin J., Pearson-Lund A., Anteau F.P., Kitaeff P., and S. Cefalu. 2021. Progress toward complete life-cycle culturing of the endangered sunflower star, Pycnopodia helianthoides. The Biological Bulletin: 241(3), 243-258.
Leahy, S. 2018, April 27. Why Europe’s Insecticide Ban Is Big News For Bees. National Geographic. https://www.nationalgeographic.com/science/article/neonics-neonicotinoids-banned-european-union-protect-bees-pollinators-environment-science-spd/
Miner C M., Burnaford J.L., Ambrose R.F., Antrim L., Bohlmann H., Blanchette C.A., … and P.T. Raimondi. 2018. Large-scale impacts of sea star wasting disease (SSWD) on intertidal sea stars and implications for recovery. PLoS One: 13(3), e0192870.
Schmidt T.S., Miller J.L., Mahler B.J., Van Metre P.C., Nowell L.H., Sandstrom M.W., … and P.M. Bradley. 2022. Ecological consequences of neonicotinoid mixtures in streams. Science Advances: 8(15), eabj8182.
Strathmann, R.R. 2014. Culturing larvae of marine invertebrates. Developmental biology of the sea urchin and other marine invertebrates: Methods and protocols, 1-25.